C. elegans can be immobilized for imaging by applying anesthetics, gluing to a substrate, or clamping in microfluidic channels, each method having its own advantages and disadvantages. Here we describe a protocol for immobilizing worms using agarose and polystyrene beads. Our method has the advantages of being inexpensive and technically simple, does not expose animals to toxic substances, and allows recovery of the animals.
1. Prepare pads composed of 10% agarose in M9 via standard methods (see Methods in Cell Biology). The solution will be too viscous to pipette. To transfer agarose, scoop with a small spatula.
2. Add 0.25-0.5 µl of 0.1 µm diameter polystyrene microspheres (e.g. Polysciences 00876-15, 2.5% w/v suspension) onto the pad.
3. Transfer worm(s) of any developmental stage to the pad using a platinum wire or eyelash pick.
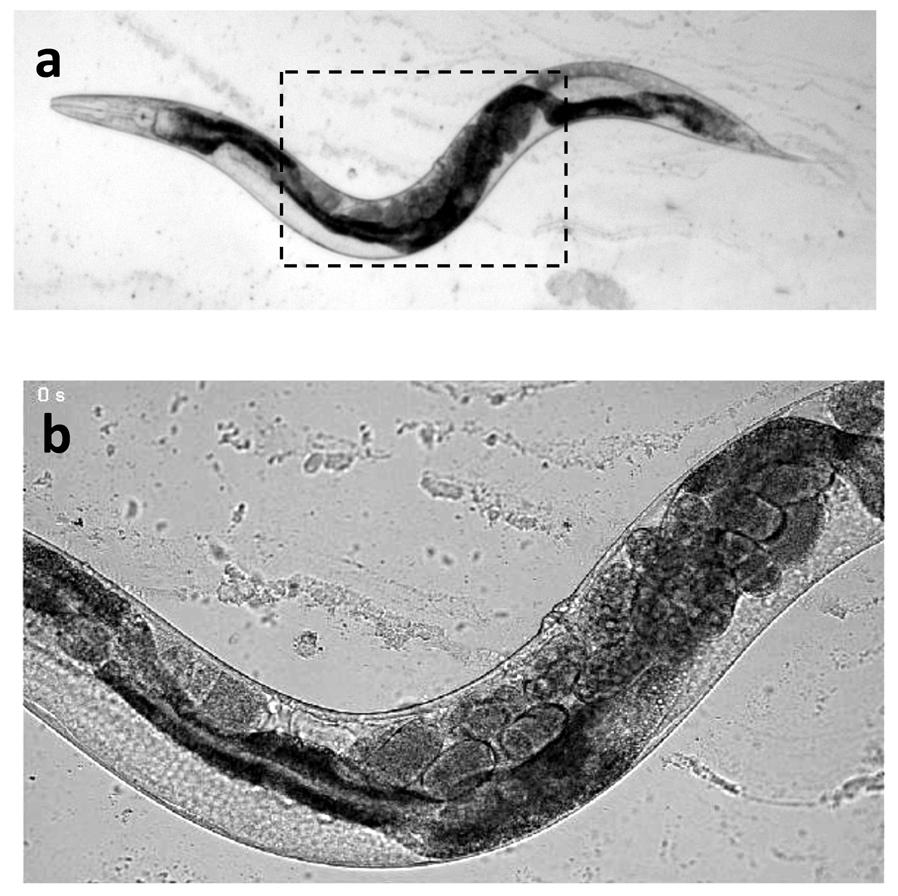
4. Gently cover with a coverslip. The worms are ready for imaging (Fig. 1).
5. To recover worms, lift the coverslip without sliding. Add a small amount of M9 to a worm and remove by pick or pipette.
How does it work? The maximum force resisting the worm’s movements is the product of (i) the normal force compressing the worm between the pad and coverslip and (ii) the friction co-efficients between the worm, agarose pad, and coverslip. The stiffer agarose pad increases the normal force (relative to ~2% agarose pads that are typically used for imaging), while the polysty-rene beads may increase the friction coefficients.
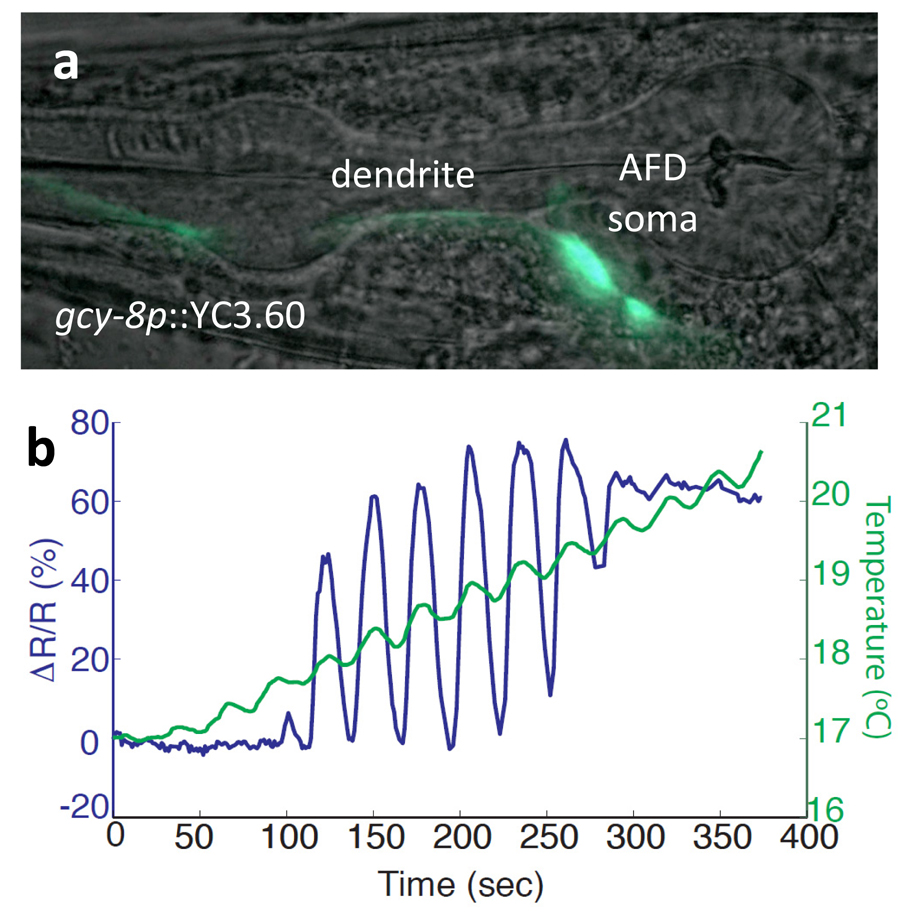
We have used agarose immobilization for fluorescence imaging, laser killing of neurons, laser cutting of axons, and calcium imaging of neurons (Fig. 2). Worms can be immobilized for many hours with no apparent harm if coverslips are sealed with wax to prevent dehydration.