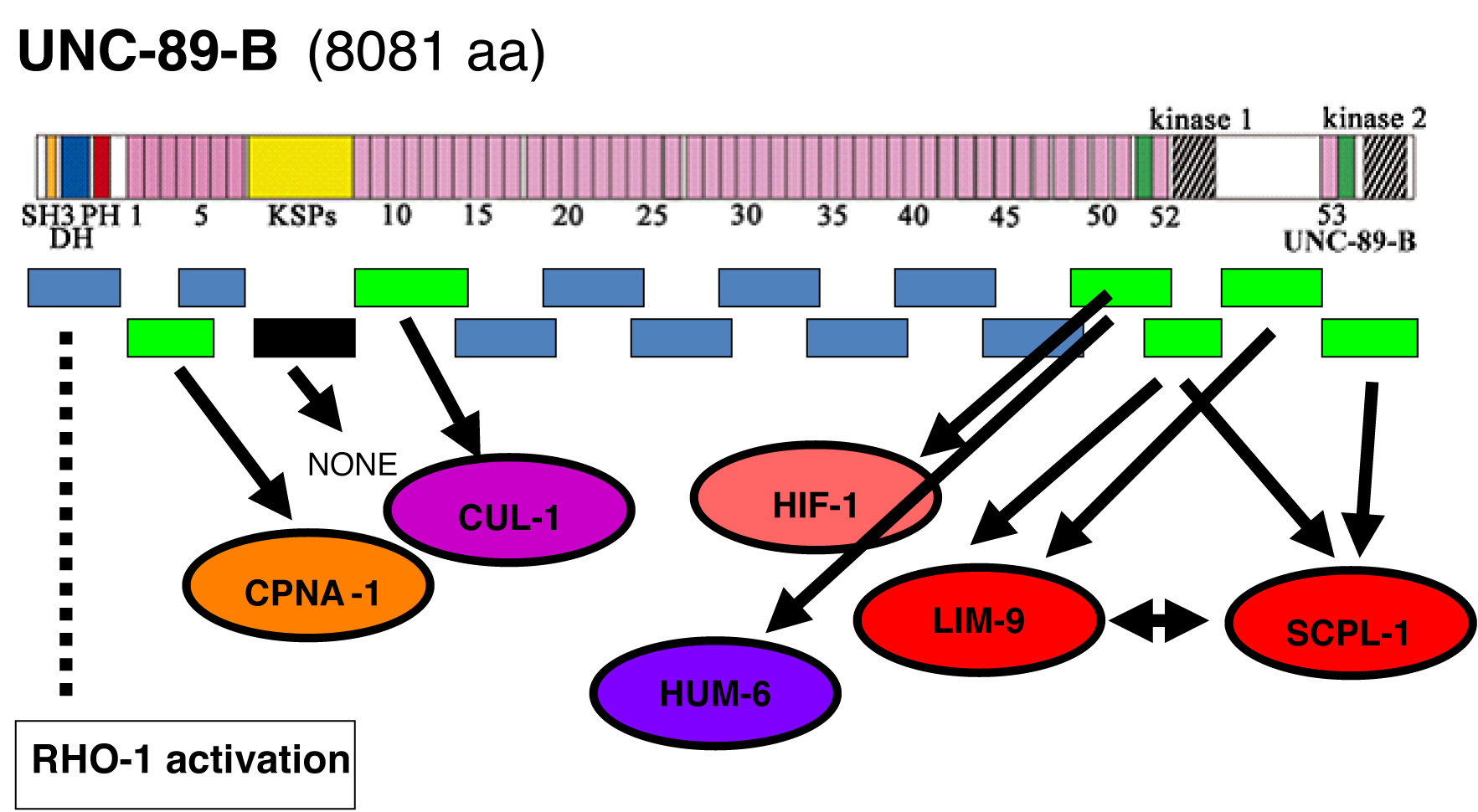
unc-89 mutants display disorganization of muscle A-bands, and usually lack M-lines. unc-89 encodes 6 major polypeptides, ranging in size from 156,000—900,000 Da. The largest of these isoforms, UNC-89-B consists of 53 Ig domains, 2 Fn3 domains, a triplet of SH3, DH and PH domains at its N-terminus, and 2 protein kinase domains (PK1 and PK2) at its C-terminus. The human homolog is called “obscurin”. Antibodies localize UNC-89 to the M-line. To clarify how UNC-89 performs its functions, we are using a yeast 2-hybrid approach to identify its binding partners. The coding sequence for UNC-89-B is entirely represented as a set of 16 overlapping segments, in both 2-hybrid bait and prey vectors. All 16 have been used to screen our yeast 2-hybrid bookshelf of approximately 23 known components of the nematode M-line, and also MHC A and MHC B. Seven of the 16 have been used to screen a yeast 2-hybrid library (RB2, kindly provided by R. Barstead). Interactions are summarized in the figure below. Previously, we reported that the predicted active kinase domain, PK2, and the predicted inactive kinase domain, PK1, each interact with SCPL-1, a CTD-type protein phosphatase. We have also reported that both the PK1 region and the 645 residue “interkinase region” each interact with LIM-9, the closest worm homolog of the human protein called FHL (“four and a half LIM domains” protein). SCPL-1 and LIM-9 have also been localized at least partially to M-lines. We have also found that SCPL-1 interacts with LIM-9. In addition, we have shown that the DH-PH region of UNC-89 activates RHO-1 (RhoA) specifically, and attenuated RNAi for rho-1 results in disorganization of muscle thick filaments.
Recently, we have determined that the segment Ig49-Ig50-Ig51 interacts with HIF-1 (hypoxia inducible factor), and the neighboring segment Fn1-Ig52 interacts with HUM-6, a class VII unconventional myosin. We have shown that HUM-6 also interacts with many of the M-line and dense body proteins (e.g. UNC-98, UNC-96, UNC-95, LIM-8 etc.). Near the N-terminus of UNC-89, we have found that Ig1-Ig2-Ig3 interacts with CPNA-1, a copine domain containing protein (Xiong et al., this issue of WBG). Finally, we have recently found that Ig8-Ig13 interacts with CUL-1 (cullin). All the interactors, except for CUL-1, interact specifically with the segments indicated in the schematic. We plan to confirm the interactions of these new proteins with UNC-89, using purified proteins. We also plan to localize the new interactors using antibodies. In the case of HIF-1, we have found that monoclonal antibodies to HIF-1 (kindly provided by Jo Anne Powell-Coffman and Mark Roth) localize to both M-lines and I-bands. Ultimately, we would like to use the remaining 9 segments of UNC-89-B to find all the interactors of this big whale!