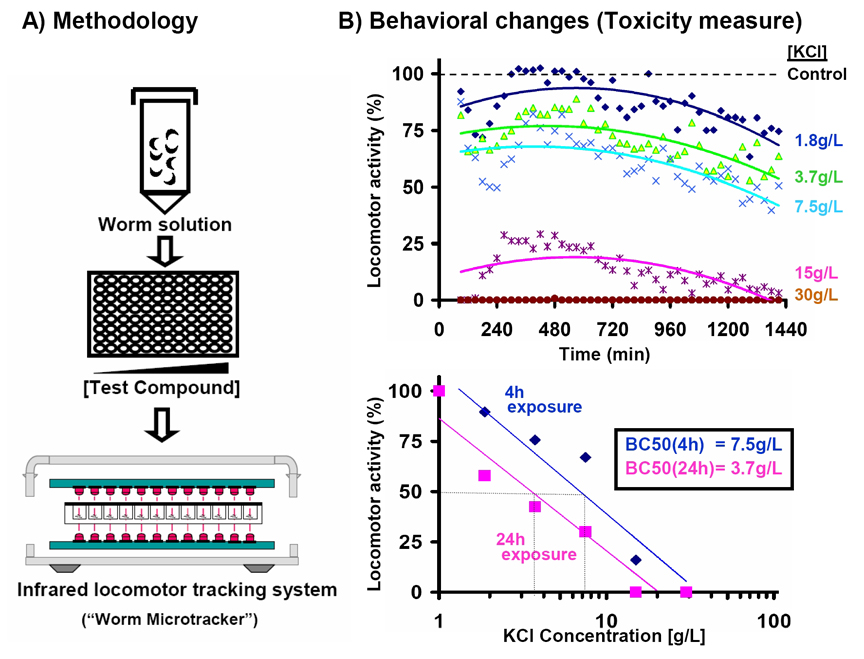
In toxicity and pharmacological studies C. elegans are exposed to different compounds and the effects analyzed by observing worm viability or behavior. An alternative approach is to use locomotor activity as a readout, which has been instrumental for assessing compound toxicity (Dhawan et al., 2000). Data acquisition usually requires microscope observation and manual counting, which is time consuming and inconvenient for extensive compound screenings. We have previously developed an automated method to detect C. elegans movement in which swimming worms are detected as they cross through infrared microbeams (Simonetta et al., 2007).
We are currently adapting this methodology for high throughput analyses. We have developed a 384 channel apparatus and successfully recorded the behavioral changes produced by toxic compounds (Figure 1). The effect increases with compound concentration and is dependent on exposure time. Our "Worm Microtracker" might be useful for the community to develop easier and faster toxicity and paralysis assays, opening the possibility of performing high throughput studies in C. elegans.
Figures
References
Dhawan R, Dusenbery DB, Williams PL. (2000). Comparison of metal-induced lethality and behavioral responses in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 19, 3061-3067.
imonetta SH and Golombek DA. (2007). An automated tracking system for Caenorhabditis elegans locomotor behavior and circadian studies application. J. Neurosci. Methods 161, 273-80. 




