The apparent simplicity and uniformity of the C. elegans nervous system belies a rich diversity of putative signaling molecules. In addition to the classical neurotransmitters, the C. elegans genome harbors a wide variety of bioactive peptides (flp, nlp and ins). Neuropeptides represent far and away the most abundant signaling molecules in the C. elegans nervous system and most of them are thought to function through the activation of G protein-coupled receptors. At the 18th International C. elegans Meeting, 2011, many presentations mentioned the involvement of neuropeptidergic signaling pathways in their field of research but hardly any data was presented on the biochemical coupling between the receptors and their peptide ligands. Despite the general knowledge and repeated predictions of peptide GPCRs following the elucidation of the C. elegans genome in 1998, only a handful of these have been deorphanized so far. Over the years, we have specialized in the identification of receptor-ligand couples and have developed an elegant way to maximize the chances of a successful receptor deorphanization using a combined reverse pharmacology approach.
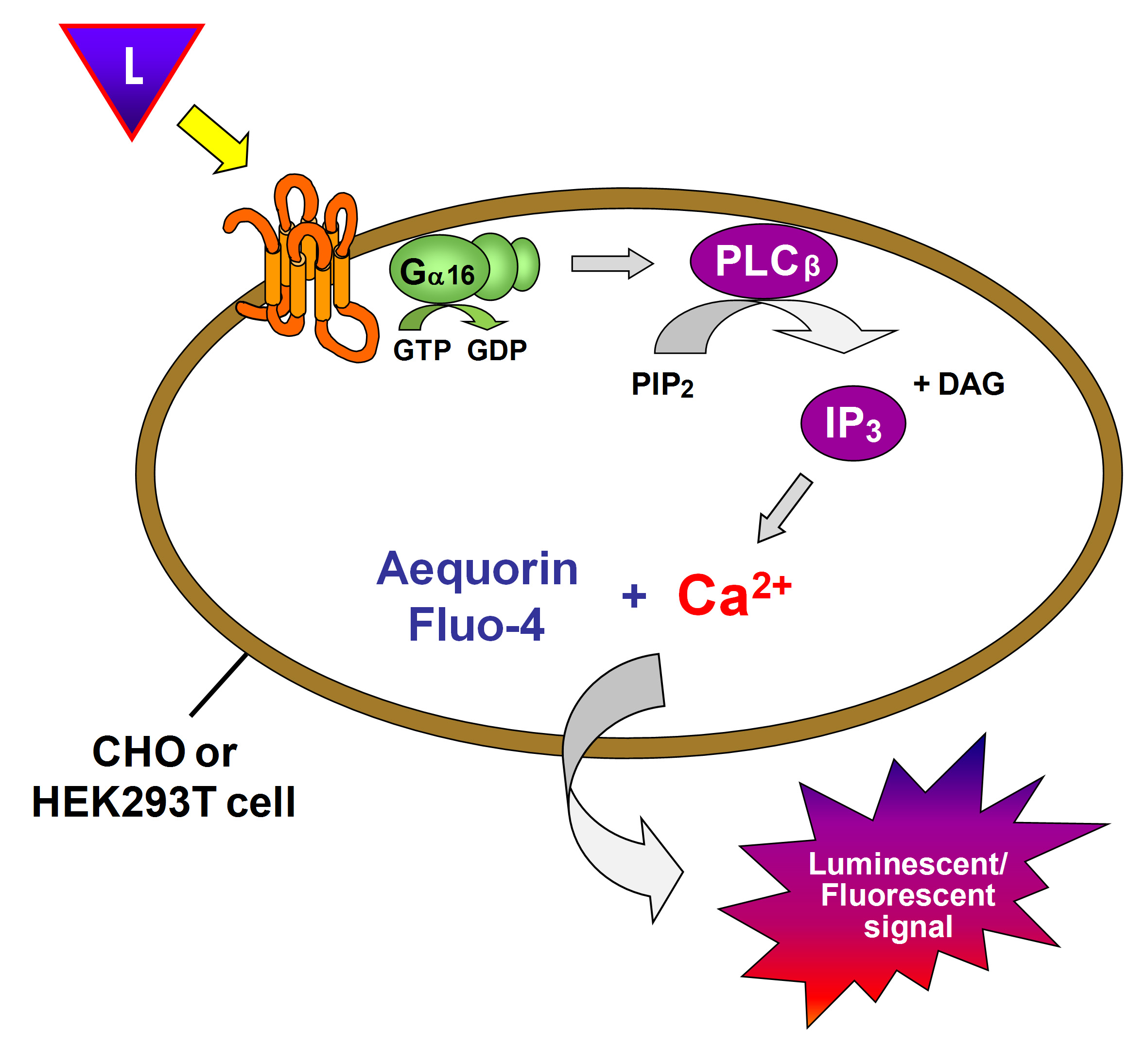
First, sequence specific primers are designed and used to amplify, clone and verify the open reading frame of the GPCR of interest. The high-level eukaryotic expression vector pcDNA3.1TM (Invitrogen) is used to generate high recombinant expression in mammalian cells driven by the human cytomegalovirus (CMV) promoter. This construct is then transiently expressed in Chinese Hamster Ovary (CHO-K1) cells and/or Human Embryonic Kidney (HEK293T) cells coexpressing the promiscuous G-alpha16 subunit, which directs intracellular signaling to a calcium flux regardless of the endogenous G protein coupling of the receptor of interest. The resulting calcium flux upon receptor activation is monitored using the calcium sensitive photoprotein aequorin or the fluorescent calcium indicator Fluo-4 (Figure 1).
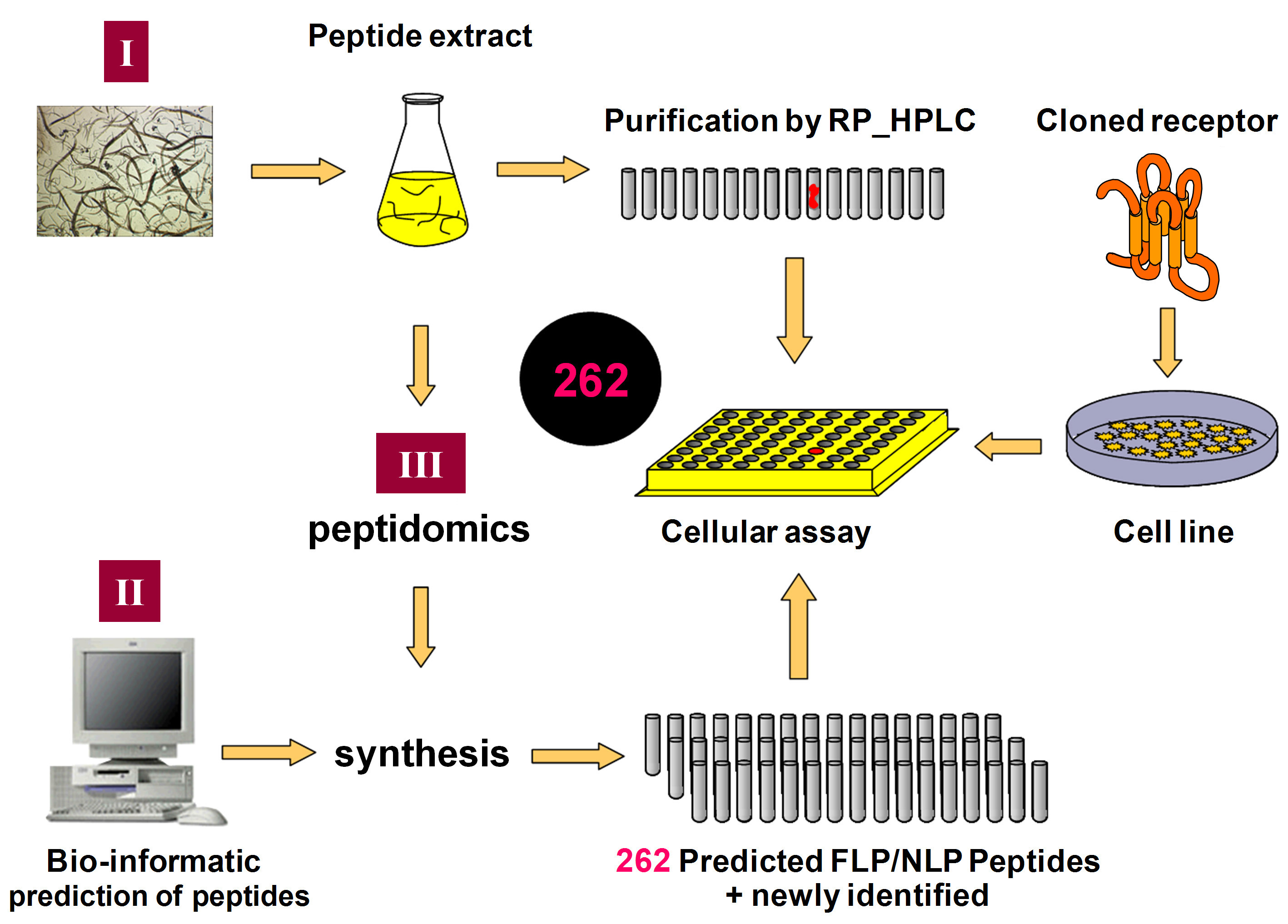
To identify the cognate ligand, we use a combination of three reverse pharmacological approaches: the tissue extract-based, the library-based and the information-based approach (Figure 2). For the tissue extract-based approach, a peptide extract from approximately 30,000,000 whole body mix stage worms was made and then subjected to reversed-phase HPLC fractionation. In addition to their direct use in the cellular pharmacological assays, the resulting peptide fractions were also studied in detail by mass spectrometric analysis (MALDI-TOFand ESI-Qq-TOFMS/MS Mass Spectrometry) to identify potential new peptides of interest. Based on bio-informatic predictions (“library-based approach”) and the mass spectrometric analysis of the reversed-phase HPLC fractions (“information-based approach”), a collection of 262 peptides, belonging to the established FLP and NLP families of peptides, was selected and custom synthesized. The peptides were selected in such a way that maximizes the number of different potential activating ligands in the combined library of synthetic and natural (endogenous) peptides (Figure 2). This combined approach enables us to test more than 262 putative peptide ligands of C. elegans for activation of orphan GPCRs.
This strategy has proven its worth as we have already uncovered more than a dozen C. elegans neuropeptide signaling systems in this way, including pigment dispersing factor (PDF), cholecystokinin (CCK) and gonadotropin releasing hormone (GnRH) signaling systems amongst others.
Figures
References
Beets I, Lindemans M, Janssen T and Verleyen P. (2011). Deorphanizing G protein-coupled receptors by a calcium mobilization assay. Methods Mol. Biol. 789 (in press)
Janssen T, Husson SJ, Lindemans M, Mertens I, Rademakers S, Ver Donck K, Geysen J, Jansen G and Schoofs L. (2008b). Functional characterization of three G protein-coupled receptors for pigment dispersing factors in Caenorhabditis elegans. J. Biol. Chem. 283, 15241-15249. 
Janssen T, Meelkop E, Lindemans M, Verstraelen K, Husson SJ, Temmerman L, Nachman RJ and Schoofs L. (2008a). Discovery of a cholecystokinin-gastrin like signaling system in nematodes. Endocrinology 149, 2826-2839. 
Lindemans M, Janssen T, Husson SJ, Meelkop E, Temmerman L, Clynen E, Mertens I and Schoofs L. (2009a). A neuromedin-pyrokinin-like neuropeptide signaling system in Caenorhabditis elegans. Biochem Biophys Res Commun. 379, 760-764. 
Lindemans M, Liu F, Janssen T, Husson SJ, Mertens I, Gäde G and Schoofs L. (2009b). Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 106, 1642-1647. 
Mertens I, Meeusen T, Janssen T, Nachman R and Schoofs L. (2005). Molecular characterization of two G protein-coupled receptor splice variants as FLP2 receptors in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 330, 967-974.