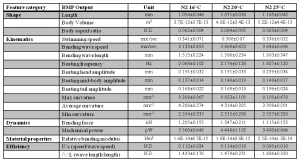
Locomotion is a fundamental phenotypic metric in C. elegans. In order to fully characterize locomotory phenotypes, various automated nematode tracking algorithms and software have been proposed and built (Buckingham and Sattelle, 2009; Fang-Yen et al., 2010; Pierce-Shimomura et al., 2008; Tsechpenakis et al., 2008). While each of these platforms has contributed in a significant way to our understanding of locomotion, a simple, quantitative, and universal tool for measuring multiple parameters of C. elegans locomotion is still lacking. In order to make characterizing locomotory phenotypes more precise, standardized and easier we integrated non-invasive video microscopy, MATLAB-based image analysis algorithms, and fluid mechanics principles into a Biomechanical Profiling Platform (BMP) to quantify C. elegans locomotion (Figure 1). Using BMP we can quantify 18 distinct features that describe C. elegans body shape, swimming patterns (kinematics) and tissue material properties (biomechanics) (Figure 2). In many cases, standard assays of motility are insufficient to describe motility defects, whereas multi-parametric comparisons reveal clear differences.
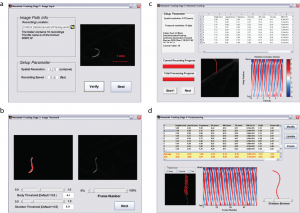
To make our integrated platform accessible to all users regardless of computational background, we derived a simple user interface for automated analysis of swimming movies (Figure 3). After defining the location of a family of sequentially named .tif images (multiple independent recordings can be analyzed and summary statistics are reported), the acquisition parameters (i.e., spatial resolution (µ/pixel) and recording speed (frames per second) are entered (Figure 3a). Users can adjust body and skeleton threshold values to obtain the best fit to their recording conditions (Figure 3b). After definition, users see the progress of analysis, including summary kinematics, biomechanics, skeletonization, and curvature plots (Figure 3c). Upon completion of the analysis, summary statistics for each of the 18 parameters are shown (Figure 3d) and users can review each individual recording for accuracy using trajectory plots, curvature analysis, and/or a skeleton browser. Data are output as .txt files for further analysis.
We have developed a free, user-friendly, and quantitative solution for integrative analysis of C. elegans locomotion. Our method uses equipment commonly found in C. elegans research labs, making it easy and inexpensive to implement. The ability to quantitatively analyze phenotypes regardless of image acquisition methods should allow comparison of data between laboratories. Full manuscript, BMP MATLAB scripts as well as detailed instructions and sample files (Krajacic et al., 2012) are available for download at www.genetics.org.
Figures

References
Buckingham SD, and Sattelle DB. (2009). Fast, automated measurement of nematode swimming (thrashing) without morphometry. BMC Neurosci. 10: 84. 
Fang-Yen C, Wyrat M, Xie J, Kawai R, Kodger Tl, Chen S, Wen Q, and Samuel AD. (2010). Biomechanical analysis of gait adaptation in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A 107, 20323-20328. 
Krajacic P, Shen X, Purohit PK, Arratia P, and Lamitina T. (2012). Biomechanical profiling of C. elegans motility. Genetics, May 2 (Epub ahead of print). 
Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, and McIntire SL. (2008). Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc. Natl. Acad. Sci. U S A 105. 20982-20987. 
Tsechpenakis G, Bianchi L, Metaxas D, and Driscoll MA. (2008). A novel computational approach for simultaneous tracking and feature extraction of C. elegans populations in fluid environments. IEEE Trans Biomed Eng. 55, 1539-1549.