The prevalence and severity of Alzheimer’s disease (AD) are influenced by gender such that two thirds of AD patients are women. However, the biological mechanisms underlying these sex differences are not fully understood. AD begins with slight memory loss and confusion, and eventually leads to severe mental impairment (Mc Khann et al., 1984). It has been previously shown by Guillozet et al. that the formation of amyloid plaques and neurofibrillary tangles in the brain are responsible for memory and other cognitive functions (Guillozet et al., 2003). A recent human trial of AD treatments has proven to be largely unsuccessful. A probable reason is that multiple factors such as food, social relationships, physical activity, gender, climate and stress all play a role in its pathology. One of the most important factors is gender (Viña and Lloret, 2010). Lack of biological gender model systems for AD limits our understanding of the role that gender play in AD pathogenesis. Elucidating the role of gender in an AD model is crucial to prevention, diagnosis, and treatment of AD in different sexes.
In order to examine the role of gender on the etiology of AD, a C. elegans model was used to emulate the pathological processes of AD. The worm apl-1 gene encodes two identical isoforms, orthologous to the human amyloid precursor protein (APP) involved in AD (Wentzell and Kretzschmar, 2010). Recently, a transgenic animal (CL4176) expressing Aβ42 in muscle tissues (Drake et al., 2003) has been established as a model. Interestingly, the Aβ protein expressed in C. elegans self-aggregates like Aβ1–42, and forms fibrillar structures (Link, 1995).
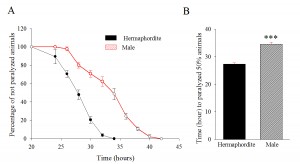
We used male and hermaphrodite CL4176 transgenic animals and performed a paralysis assay (Drake et al., 2003). Synchronized eggs were maintained at 16 ºC and males and hermaphrodites were sorted at late L4 stage. Sorted worms were subjected to 25 ºC to induce the transgene expression and the paralysis was scored successively at 2 hour intervals. The hermaphrodite animals paralyzed earlier in comparison to the males. The mean time duration at which 50% worms were paralyzed was 34.6 hours and 27.3 hours, for males and hermaphrodites, respectively (p< 0.001) (Figure 1A, 1B). The data shows that the male CL4176 takes significantly more time to undergo paralysis compared to the hermaphrodites.
In this paper, we provide a way to study the mechanism and treatment strategy of AD disease based on gender. The observed phenotype is consistent with human beings (Viña and Lloret, 2010). Further studies will allow us to investigate the molecular pathways involved in AD in relation to sex and significantly impact our understanding to identify novel therapeutics in the future.
Figures
References
Drake J, Link CD, and Butterfield DA. (2003). Oxidative stress precedes fibrillar deposition of Alzheimer's disease amyloid beta-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 24, 415-420. 
Guillozet AL, Weintraub S, Mash DC, and Mesulam MM. (2003). Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch. Neurol. 60, 729-736. 
Link, CD. (1995). Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 92, 9368–9372. 
McKhann G, Drachman D, Folstein M, Katzman R, Price D, and Stadlan EM. (1984). Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease, Neurology 34, 939-944. 
Viña J, and Lloret A. (2010). Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J. Alzheimers Dis. S527- 533. 
Wentzell J, and Kretzschmar D. (2010). Alzheimer's disease and tauopathy studies in flies and worms. Neurobiol. Dis. 40, 21–28. 




