During larval development C. elegans has two distinct time points where it can arrest development until environmental conditions become more favorable. The first one is L1 arrest, which occurs when worms hatch in the absence of food. The second decision point is later, around L1-L2 molt, and if conditions are poor at this time (little food, crowding, and high temperature) worms gain the potential to become dauers. L1 arrest and dauer formation are not mutually exclusive and, in principle, a worm can go through both of them sequentially. However, it is not known whether L1 arrest and dauer formation are coupled, i.e., if experiencing L1 arrest affects a worm’s decision to become a dauer. A naïve prediction would be that worms that experienced L1 starvation should be more susceptible to become dauers since they were already exposed to a harsh environment.
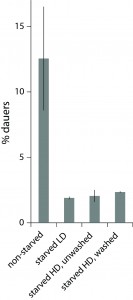
We studied dauer formation of N2 worms in liquid and found that in reality worms that experienced L1 arrest and starvation were less likely to become dauers. In a typical experiment, eggs obtained from an egg prep were incubated at 20 0C in S-complete with a small amount of bacterial food (HB101) and dauer formation was assessed 6 days later based on survival in 1% SDS (Pungaliya et al., 2009). When we let eggs hatch without food and added it 1-4 days later, keeping other conditions the same, significantly fewer worms became dauers (Figure 1). This was not due to slower development after starvation and incomplete dauer formation. We also found that this effect reflected an internal state of the worm rather than exposure to L1 exometabolome during L1 arrest since worms starved as L1s at low and high density later formed dauers with the same probability (Figure 1, during the dauer formation worms were at the same low density of 1 worm/µl to minimize the effect of released chemicals, see below).
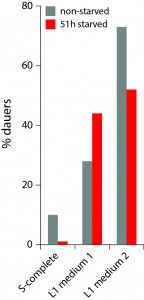
Starved L1 worms release numerous metabolites, including several ascarosides, and we anticipated that they might promote dauer formation. Indeed, when S-complete was substituted with conditioned medium from high-density L1 larvae starved for 24 h, more worms became dauers (Fig. 2), consistent with the known dauer-inducing effect of crowding mediated by ascarosides (Ludewig and Schroeder, 2013). However, this result apparently contradicts the starvation effect described above. On one hand, when a worm itself experienced L1 arrest but received no signal from other worms (low density condition), it was less likely to become a dauer and preferred to try to grow to adult. On the other hand, a worm that had not experienced starvation itself but was exposed to chemical signals released by other starved L1 worms, was more likely to become a dauer. The next obvious experiment is to combine these two opposite effects and see which one is stronger. The result of this test (Fig. 2) is that the external starvation signal (mediated by L1 conditioned medium) overrides the internal one (L1 arrest). When making a decision whether to become a dauer, apparently C. elegans worms trust the communal voice more than their own.
While the dauer-inducing effect of L1-conditioned medium is intuitively easy to accept, the negative effect of L1 starvation on subsequent dauer formation is less clear. A recently published model of contrast effects (McNamara et al., 2013) may provide a theoretical framework for understanding this phenomenon. But to keep us from being too comfortable with our simplistic views nature presented us with another puzzle: L1 arrest does not negatively affect dauer formation in C. briggsae.
Figures
References
Ludewig AH and Schroeder FC. (2013). Ascaroside signaling in C. elegans (January 18, 2013), WormBook, ed. The C. elegans Research Community. 
McNamara JM, Fawcett TW, and Houston AI. (2013). An adaptive response to uncertainty generates positive and negative contrast effects. Science 340, 1084-1086. 
Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, and Schroeder FC. (2009). A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc. Natl Acad. Sci. U. S. A. 106, 7708-7713. 




