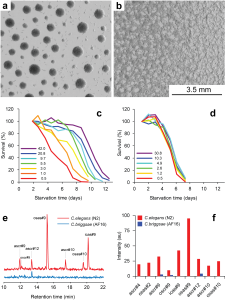
Working with starved L1 larvae of C. elegans and C. briggsae we noticed that these two species behave quite differently in starvation. First, C. elegans adults stop laying eggs after exhausting bacterial food, which eventually leads to internal hatching and bagging. C. briggsae do not show this behavior. This difference has been observed before (McCulloch and Gems, 2003). Second, at high enough density of worms, arrested C. elegans L1s aggregate on agar plates after several days of starvation (Fig. 1a). C. briggsae L1s do not form aggregates (Fig. 1b). Aggregation may serve several purposes ranging from decrease of surface to volume ratio and use of diffusible “public goods” to sharing information about quality of the environment. Third, survival of starved C. elegans L1s strongly depends on their density – the higher the worm density, the longer they survive (Fig. 1c) (Artyukhin et al., 2013). This holds true for starvation on plates as well as in suspension. Survival of C. briggsae L1s is independent of worm density (Fig. 1d). We believe that the aggregation and the density dependence are naturally connected as they seem to be correlated across Caenorhabditis species. Forth, starved C. elegans L1s release plenty of ascarosides (Fig. 1e,f). C. briggsae L1s release far less, both in variety (Fig. 1e) and in amounts (Fig. 1f). More than 100 ascarosides have been found in C. elegans (von Reuss et al., 2012), and although we do not know physiological functions for many of them, functions of the ones that we do know and the high degree of structural conservation in other nematodes suggest that ascarosides constitute a vital part of nematode chemical language. Hence, we interpret the data in Fig. 1e,f as suggesting that C. elegans L1s “talk” more with each other in starvation than C. briggsae. Finally, C. elegans is one of a couple Caenorhabditis species susceptible to environmental RNAi (Winston et al., 2007), which has been speculated to play a role in communication between organisms (perhaps, conspecifics) (Whangbo and Hunter, 2008). Based on everything above, we speculate that in unfavorable conditions (starvation) C. elegans tend to become more social than C. briggsae. In variable and unpredictable environments genotypic fitness can be maximized either by reducing individual-level variance in fitness or by reducing between-individual correlations in fitness (or some combination of the two) (Starrfelt and Kokko, 2012). Aggregating or social species may choose to minimize individual-level variance by having a mechanism that helps them to adjust to unfavorable conditions, in part through collective behavior. Non-aggregating species may use dispersal as a strategy to minimize between-individual correlations. Based on this hypothesis we would predict that lack of aggregation and density dependence in C. briggsae implies that their starved L1s hardly ever find themselves at high density in nature and the optimal strategy for them is to disperse and actively look for food.
Figures
References
Artyukhin AB, Schroeder FC, and Avery L. (2013). Density dependence in Caenorhabditis larval starvation. Sci. Rep. 3, 2777. 
McCulloch D and Gems D. (2003). Evolution of male longevity bias in nematodes. Aging Cell 2, 165-173. 
Starrfelt J and Kokko H. (2012). Bet-hedging - a triple trade-off between means, variances and correlations. Biol. Rev. 87, 742-755. 
Whangbo JS and Hunter CP. (2008). Environmental RNA interference. Trends Genet. 24, 297-305. 
Winston WM, Sutherlin M, Wright AJ, Feinberg EH, and Hunter CP. (2007). Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc. Natl. Acad. Sci. U. S. A. 104, 10565-10570. 
von Reuss SH, Bose N, Srinivasan J, Yim JJ, Judkins JC, Sternberg PW, and Schroeder FC. (2012). Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J. Am. Chem. Soc. 134, 1817-1824.