Many species of nematodes are known that are pathogenic as well as toxigenic for plants and many of them live as a parasite on the host plant causing severe crop damage and economic loss (Burglin et al., 1998). Although chemicals are available to control the harmful nematodes, they have their own set of deleterious effects such as decrease in soil fertility, impact on non target beneficial fauna, decrease in crop yield, and toxic effects on farmers. As fungi are known to have nematocidal activity (Khan et al., 2003), they may be used as a potential biological control agent.
In order to examine the effect of extra-cellular fungal extract as a nematicidal agent, C. elegans was used as a model system. In this study, we choose two Indian fungal isolates: (1) Aspergillus terreus, and (2) Fungal sp. US14, for its nematicidal potential on C. elegans. Fungal sp. US14 showed only 96% sequence similarity with Purpureocillium lilacinum with regards to its internal transcribed spacer 1 (ITS1), 5.8S ribosomal RNA gene and internal transcribed spacer 2 (ITS2) sequences. So, it might be a new species and was deposited at NCBI in the name of Fungal sp. US14 (NCBI accession number-JN802258.1).
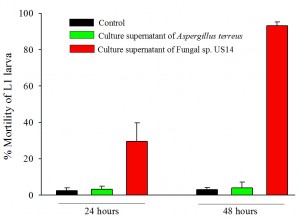
We carried out mortality assays on C. elegans using seven-day old grown-culture supernatant (extra-cellular culture filtrate) of both fungi. Synchronised L1 larvae of wild-type C. elegans were transferred with and without fungal supernatants, followed by incubation at 15oC. Mortality % of larvae was determined up to 48 hours by counting the dead larvae in the presence of fungal supernatant as compared to control animals which were grown in M9 buffer. We counted the number of dead larvae at 24 hours and 48 hours. The fungal supernatant of US14 killed 29±10.5% larvae at 24 hours and 93±1.9% at 48 hours (p<0.0001) compared to control larvae. The fungal supernatant of Aspergillus terreus did not kill larvae (Figure 1), and was almost similar to the control assay, suggesting its nonpathogenecity towards C. elegans.
Some of the study shows that the nematicidal activity of the fungus may be attributed to the presence of chitinase, protease, and lipases in the culture supernatant. The chitinase activity destroys the cuticle of nematodes and causes mortality (Miller and Sands, 1977). Furthermore, chitinase activity of nematophagous fungi has been previously correlated with their pathogenicity to some plant-parasitic nematodes (Stirling and Mankau, 1979). All together, the Fungal sp. US14 appeared as a promising candidate for biological control of nematodes.
Figures
References
Bürglin TR, Lobos E, and Blaxter ML. (1998). Caenorhabditis elegans as a model for parasitic nematodes. Int. J. Parasitol. 28, 395-411. 
Khan A, Williams K, and Nevalainen H. (2003). Testing the nematophagous biological control strain Paecilomyces lilacinus 251 for paecilotoxin production. FEMS Microbiol. Lett. 227, 107-111. 
Miller, PM and Sands, DC. (1977). Effects of hydrolytic enzymes on plant-parasitic nematodes. J. Nematol. 9, 192-197. 
Stirling, GR and Mankau, R. (1979). Mode of parasitism of Meloidogyne and other nematode eggs by Dactylella oviparasitica. J. Nematol. 11, 282-288.